Removing A Medtronic Pain Pump
Position the pump and catheter. Had Medtronic intrathecal pain pump implanted in January 1998 by Dr.
About Your Medtronic Implanted Infusion Pump Memorial Sloan Kettering Cancer Center
With reprogramming and refill 11405 9160 Electronic analysis of pump.

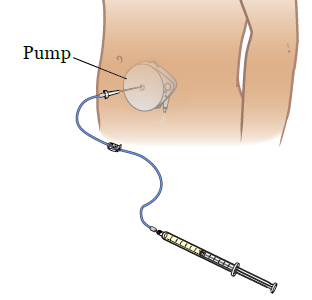
Removing a medtronic pain pump. Pre-sterilized kit contains needle and all necessary instruments to insert catheter in cerebrospinal fluid. Cost of Programmable pump is higher than non-programmable pump. It is important to know that the SynchroMed II pump will last a maximum of 7 years. The pump may be refilled with drugs normally spasticity medication such as Baclofen or painkillers by a clinician or doctor with a non-invasive injection. I cant say enough GOOD things about. Pump Removal78 0JPT0VZ Removal of infusion pump from trunk subcutaneous tissue and fascia open.
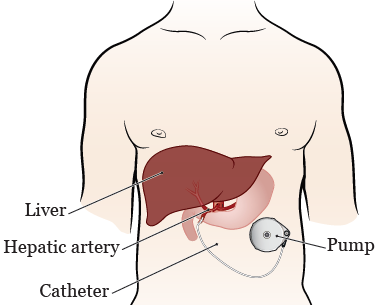
Pain Spine Orthopaedic Urinary Womens Health Support Back to Support Contact Patient Services Electromagnetic Compatibility Guide for Cardiac Devices Health Monitoring. Indicates that the SynchroMed EL infusion system also known as a Medtronic intrathecal pain pump is an implantable programmable drug delivery system that uses a catheter to deliver precise amounts of pain medication or intrathecal baclofen a muscle relaxant and antispasticity agent directly to the spinal cords. The automatic shut-off feature helps ensure that the pump works correctly to provide pain therapy. Intrathecal Drug Infusion System for Chronic Pain Management. Create a second incision on your back for the catheter. SynchroMed II pumps can be managed using the Control Workflow SM approach which is designed to help eliminate systemic opioids and provide effective pain relief.
Removal of subcutaneous reservoir or pump previously implanted for intrathecal or epidural infusion 29209 187790 Electronic analysis of pump. Im requesting advice in lieu of tracking down my now world-famous doctor who implanted my Medtronic Intrathecal Pain Pump. The pump should resume normal operation when removed from the MRI magnetic field but there is a potential for a delay in the return of proper drug infusion and a delay in the logging of motor stall events after an MRI in the SynchroMed II pumps. The pain the underlying condition is coded and sequenced as the principal diagnosis2. Once the pump and catheter are in place the incisions are closed and the surgery is complete. Image from Medtronic The FDA has classified the recall of Medtronics NYSEMDT SynchroMed II implantable drug pumps as Class I the.
Medtronics SynchroMed II implantable pain pump system. Medtronic designed an implantable pain pump that would deliver medication directly to the patient at pre-set intervals. The pumps are used to treat chronic pain and cancer. The dr has a responsibility of weaning you off of the medications. There are two types of intrathecal pain pump known as programmable pump and non-programmable pump. With reprogramming 5421 2924 Electronic analysis of pump.
Wreprogrammingrefill MD or OQHP 11890 8772. The cost also includes intrathecal catheter and kit. Removing the Pump If you no longer need the pump or change your mind about the pain treatment your doctor can turn it off or surgically remove the system. The pump is programmed to slowly release medication over. However reports of complications following the removal of chronically placed catheters are scarce. So it would seem to me that any doctor involved with removing a pump would have a very close working arrangement with Medtronics and would be able to get you any information you need.
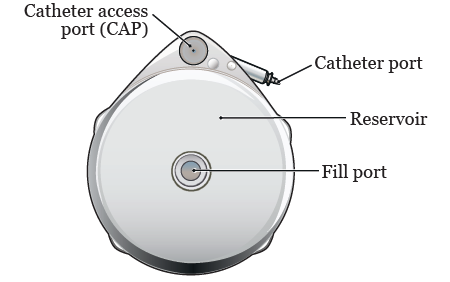
The intrathecal pump system consists of a pumpreservoir implanted between the muscle and skin of your abdomen and a catheter that carries pain medication from the pump to the spinal cord and nerves. Make an incision and form a pocket to hold the pump. If you have a SynchroMed II pump you will need to have your pump surgically replaced with a new pump before it automatically shuts off. Implanted intrathecal drug delivery systems IDDS are increasingly used in the treatment of spasticity and in patients with refractory pain. It is usually defined as pain of at least 6 months duration and can include pain from a chronic condition prolonged healing from an injury or an unknown cause. Medtronic provides this information for your convenience only.
You cannot just stop having it filled because the withdrawals from the opioids would land you in the hospital. Intrathecal Pain Pump Removal Advice. I know when I had a trial for a pump the Medtronic representative was very involved both with the doctor and with me on the phone. Chronic nonmalignant pain is a complex condition involving neurophysiologic cognitive behavioral cultural social and economic factors. Medtronic Operational Headquarters 710 Medtronic Parkway Minneapolis MN 55432-5640 USA. Literature discussing complications associated with intrathecal pump placement is widely available.
The surgery takes approximately 1 to 3 hours. Posted 8222017 614 AM GMT -7 Before having a pump removed a patient should be weaned down off of the medications used in the pump. It does not constitute legal advice or a recommendation. The SynchroMed II programmable implantable pump delivers drug to the intrathecal space via an implanted catheter. Back to top. This could have several advantages including providing consistent levels of medication and avoiding the risk of the patient accidentally over-medicating or over-dosing themselves.
The implantable Medtronic pain pump is a type of peristaltic pump that has a rotating motor that works to push medicine through a catheter tube into the patients spinal column. Medtronic is recalling its SynchroMed implantable infusion pumps due to a software glitch that could cause the devices to deliver too much or.

Medtronic Updates Troubled Synchromed Infusion Pump In Class 1 Recall Fiercebiotech

Are You A Candidate For Intrathecal Pain Pumps For Chronic Pain
About Your Medtronic Implanted Infusion Pump Memorial Sloan Kettering Cancer Center

Medtronic Pain Pump Recall Deemed Class I Massdevice

Fda Approves Medtronic S New Synchromed Ii Myptm Personal Therapy Manager Medical Product Outsourcing

Medtronic Must Face Pain Pump Lawsuit Drug Delivery Business

Guide To Implantable Devices For Intrathecal Therapy
Medtronic Issues Recall Of Some Models Of Synchromed Ii Drug Pump Star Tribune
Medtronic S Pain Pump Recall Won T Hurt The Bottom Line Analysts Say Massdevice

Intrathecal Pump Implant Medtronic Southern California Orthopedic Institute
About Your Medtronic Implanted Infusion Pump Memorial Sloan Kettering Cancer Center

Medtronic Synchromed Ii Intrathecal Pump Programmer And Patient Download Scientific Diagram

Posting Komentar untuk "Removing A Medtronic Pain Pump"